ChapterⅠ – Introduction and Application of SF6 Gas
SF6 (SF6) is made by the reaction of sulfur and fluorine at high temperatures (i.e., S+3F2→SF6). It is a colorless, odorless, and non-toxic gas at room temperature and pressure. The non-combustion thermal conductivity of SF6 is smaller than air, making it an excellent cooling medium. Its unique and stable regular octahedral molecular structure makes its chemical properties extremely inactive within the normal operating range of electrical equipment. It does not react with electrical materials such as copper, steel, and aluminum; Non-corrosiveness, inactive pharmacological properties, non-toxicity, and is slightly soluble in water. SF6 gas is widely used in the electrical industry, such as circuit breakers, high-voltage switchgear, high-voltage transformers, gas-enclosed combined capacitors, high-voltage transmission lines, and transformers. SF6 is also widely used in metal smelting, atmospheric tracing, electronics manufacturing, and other industries due to its chemical inertness, non-toxicity, non-combustibility, and non-corrosive properties
1.1 Application Advantages Of Sf6 In The Power Industry
A. Good electrical insulation performance and arc extinguishing performance. The insulation performance is 2 to 3 times that of air. The greater the gas pressure, the higher the insulation performance. At a pressure of 294.2 kPa, the dielectric strength of SF6 is roughly equivalent to that of transformer oil.
B. Superior arc extinguishing characteristics of SF6. Due to its electronegativity, the arc extinguishing performance is about 100 times that of air, which is especially suitable for high voltage and high current breaking.
C. Due to the excellent insulation performance of SF6 gas, the insulation distance of equipment is significantly reduced. Also, the area and space volume is significantly reduced. It is precise because of these excellent electrical properties that SF6 has been increasingly used in the power industry. And in recent years, it has continued to expand to medium and low-voltage applications.
1.2 Hazards of SF6 gas
1.2.1 SF6 Gas Harm
(1) Greenhouse gas: SF6 gas can exist stably in the atmosphere without being decomposed for 3200 years, and its greenhouse effect is 23900 times that of the equivalent carbon dioxide.
(2) Hazard to the human body: pure SF6 is colorless, odorless, and non-toxic, but a large amount of inhalation may cause suffocation.
1.2.2 Hazards after decomposition of SF6
Pure SF6 gas is a colorless, odorless, non-toxic, non-flammable halogen compound. Its relative density is 6.16g/cm3 (at 20°C, 0.1MPa) in the gaseous state and 1400g/cm3 (at 20°C) in the liquid state, about 5 times the relative density of air in the same state. Easy to transport and store, SF6 gas usually exists in the cylinder in liquid form. The chemical nature of SF6 gas is relatively stable. It does not burn in the air, does not support combustion, and does not react with water, strong alkali, ammonia, hydrochloric acid, sulfuric acid, etc.; when the temperature is lower than 150℃, SF6 gas is chemically inert, rarely melts in water and micro-melting in alcohol. It has no chemical effect on metals and other organic materials commonly used in electrical equipment. However, under the high-power arc, spark discharge, and corona discharge, SF6 gas can decompose and liberate various products, mainly SF4 and SF2, as well as a small amount of S2, F2, S, F, and so on.
(1) Toxicity source of SF6 gas
From relevant departments’ tests and research results, the toxicity of SF6 gas mainly comes from five aspects.
① SF6 gas is impure and contains high-toxicity low-sulfur fluoride, hydrogen fluoride, and other toxic gases when leaving the factory. As we all know, the current method of producing SF6 gas in the chemical industry is mainly formed by the direct reaction of elemental sulfur and excess gaseous fluorine; that is, S+3F2→SF6+Q (heat release). The synthesized crude product contains a variety of impurities. The composition and content of impurities vary significantly due to the purity of raw materials, the material of production equipment, process conditions, and other factors. The total content of impurities can reach 5%, which is composed of sulfur-fluorine compounds, such as S2, F2, SF2, SF4, S2F10O, etc.; sulfur-fluorinated oxygen compounds such as SOF2, SO2F2, SOF4, S2F10O, etc., and impurities carried in raw materials such as HF, OF2, CF4, N2, O2… To purify the impurities in the crude product, the synthesized SF6 gas also needs to undergo a series of purification treatments such as water washing, alkaline washing, pyrolysis (removal of highly toxic fluorides), drying, adsorption, freezing, distillation, and purification to obtain the purity 99.8% or more of the products. Then it is pressurized by a compressor and filled into a steel cylinder whose temperature is lowered to about -80℃ and is liquid. It is released under reduced pressure when used and rushing into electrical equipment in a gaseous state.
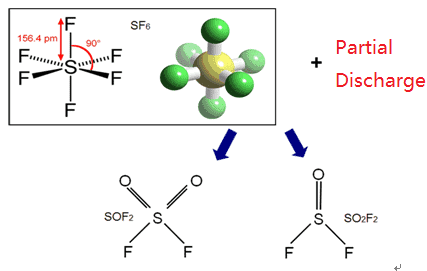
In addition to the impurities generated during the above synthesis process, a small amount of air, moisture, mineral oil, and other impurities may be mixed in the gas filling process, which all carry or produce certain toxic substances. Therefore, in order to ensure the purity and quality of SF6 products, the International Electrotechnical Commission (IEC) and many countries have established quality standards for SF6 products that leave the factory and require manufacturers to provide biological test non-toxic certificates before supplying.
② Some toxic products are produced when SF6 gas in electrical equipment acts on the high-temperature arc.
For example the decomposition of SF6 gas in the arc and the reaction with oxygen:
2SF6+O2→2SOF2+8F (sulfuryl fluoride)
2SF6+O2→2SOF4+4F (sulfuryl tetrafluoride)
SF6→SF4+2F (four Sulfuryl fluoride)
SF6→S+6F (sulfur)
2SOF4+O2→2SO2F2+4F (sulfuryl fluoride)
③ The SF6 gas decomposition product in electrical equipment reacts with the moisture inside to form some toxic products.
For example the secondary reaction of SF6 gas decomposition products and water:
SF4+H2O→SOF2+2HF (hydrofluoric acid)
SOF4+H2O→SO2F2+2HF (hydrofluoric acid)
SOF2+H2O→SO2+2HF (sulfur dioxide)
SO2F2+ 2H2O→H2SO4+2HF (sulfuric acid)
④ SF6 gas and decomposition products in electrical equipment react with electrodes (Cu-W alloy) and metal materials (AL, Cu) to generate certain toxic products.
For example SF6 gas and decomposition products react with electrodes or other materials:
3SF6+W→WF6 (gaseous)+3SF4
3F+AL→ALF3 (solid powder)
3SOF2+AL2O3→2ALF3 (solid powder)+3SO2
SF6+Cu→CuF2 (Solid powder)+SF6
4SF6+W+Cu→2S2F2 (gaseous)+3WF6 (gaseous)+CuF2 (solid powder).
⑤ SF6 gas and decomposition products in electrical equipment react with insulating materials to produce certain toxic products. Such as insulating parts with epoxy phenolic glass cloth board (rods, tubes) containing silicon components; or epoxy resin casting parts, molded parts, porcelain bottles, silicone rubber, silicone grease, etc., with quartz sand and glass as fillers. , Produce SiF4, Si(CH3)2F2 and other compositions.
For a long time, due to inappropriate use and management of SF6 gas, much toxic and corrosive gases and solid decomposition products were discharged into the atmosphere, which not only caused irretrievable pollution and damage to the environment on which we depend but also endangered the normal operation of electrical equipment and people’s health. The physiological characteristics of the arc decomposition products and reaction products of SF6 gas are shown in the following table:
| NO. | Chemical Composition | Physiological Characteristics |
| 1 | WF6 | Irritating |
| 2 | S2F2 | Both mice died within 15 minutes at the concentration of 10×1000μg/g, which was irritating |
| 3 | SF4 | At the concentration of 19μg/g, the mortality of animals within 4h is 50%; Stay at the concentration of 1h at 10μg/g, the animal will have signs of breathing difficulties |
| 4 | S2F10 | Mice stayed at the concentration of 0.1μg/g for 1h without symptoms of poisoning; stimulated the lungs at the concentration of 1μg/g; decayed the lungs at the concentration of 10μg/g |
| 5 | SOF2 | Irritating |
| 6 | SO2 | Irritating |
| 7 | HF | Irritating |
| 8 | SiF4 | Irritating, dangerous to breathing after ingested from food |
Table 1 Physiological characteristics of SF6 gas arc decomposition and reaction products






